Digital Measurements to Improve Clinical Care for Kidney and Heart Patients

Project Title: REDIEM – Renal Extremity Device to Measure Impedance, Edema and Movement
Team 22054 Members:
Spencer Alexander Ciammitti, project lead, biomedical engineering
Emmanuel Gabriel Enriquez, electrical and computer engineering
Diana Kathryn Meyer, mechanical engineering
Jocelyn Reynolds, biomedical engineering
Madelyn Reynolds, biomedical engineering
Julia Mary Starkey, biomedical engineering
Sponsor: Dr Marvin Slepian/ACABI and the Kidney ADVANCE Project/NIH
Dr. Bijin Thajudeen treats people with kidney disease. When his patients experience kidney failure, fluid buildup and swelling occur because their kidneys don’t remove waste and extra water from the blood the way healthy kidneys do. Thajudeen helps patients eliminate excess fluid with medications or dialysis.
“The problem is, that’s based on a judgment I make as a physician, and I can go wrong, because each human body is different. I might be overdoing or underdoing, which could result in bad outcomes in my patients,” said Thajudeen, a mentor for team 22054. He’s also chief of the nephrology service at Banner – University Medical Center South campus and a College of Medicine – Tucson associate professor.
Patients with kidney failure, and medical professionals like Thajudeen, would benefit from a device that digitally measures the body’s water content, or impedance, and edema, or swelling, to guide more precise treatment.
The Arizona Center for Accelerated Biomedical Innovation, or ACABI, sponsored six Interdisciplinary Capstone projects this year, with three as part of the Kidney ADVANCE Project, a five-year grant funded by the National Institutes of Health to advance student interest and participation in kidney health and medicine. Team 22054 is creating a prototype renal measurement device and phone app to improve treatment.
The best place for such a device is on the leg, because that’s where gravity allows most of the excess fluid to accumulate. A measurement device on the leg could also help observe and diagnose symptoms of restless leg syndrome, known as RLS, which commonly affects the estimated 37 million American adults – 15% of the population – living with kidney disease, as well as others.
RLS can be confused with other conditions, such as cramping or nerve and muscle disorders, which sometimes leads to inefficient treatment, according to Thajudeen. And good RLS treatment is important because the condition can affect people’s ability to sleep and take part in activities.

The team built a wearable, stocking-like device that measures impedance by applying constant voltage and current to a four-electrode circuit and determining the resistance value, then extrapolating the amount of edema. An accelerometer detects movements associated with RLS motion. The data goes to an app for patients and physicians to view.
Team lead Spencer Ciammitti, a biomedical engineering major, is excited the project includes both hardware and software components.
“It’s meshing those two worlds, and it’s cool to kind of get to brag for my team about that, because our project is big and ambitious,” he said.
The team’s paper on the project has been accepted for presentation at the 67th Annual Scientific Conference of ASAIO, the American Society for Artificial Internal Organs, in June. At that meeting, the team will compete in the organization’s student design competition. The team will also present a poster at this summer’s Capstone Design Conference.
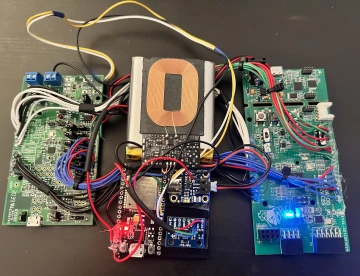
Just as important to Ciammitti, his team has prototyped a technology solution that could evolve to become a real product for the health care market and fill a need.
“This will actually, hopefully, be used one day,” he said. “It's really cool to think this could go on someone's leg and give quantifiable feedback and understanding on the at-home side.”
Next year, another capstone team will refine the prototype built by this group, said Dr. Marvin Slepian, the project sponsor and additional mentor, who is associate department head for biomedical engineering clinical and industrial affairs. And, thanks to the NIH funding, additional undergraduate students will continue work on the project at ACABI with Slepian, who is also the center’s director and a Regents Professor of materials science and engineering and at the BIO5 Institute.
The renal extremity device will likely be classified as a 510(k) or medium-risk device, Slepian said. This means the Food and Drug Administration regulatory pathway is less complex than, for example, an implantable device. Slepian estimates a full market launch would take around three years in total.
In addition to the need it meets for kidney patients, the project could help those experiencing heart failure and other disease states characterized by edema and swelling.
“This potential product could guide the patient and cardiologist in managing excessive fluid accumulation, which when out of balance leads to a congested state,” Slepian said.
This is Thajudeen’s first time serving as an Interdisciplinary Capstone mentor, and he’s pleased to work with engineering students on trying to improve current diagnostic methods.
“This is a good step in the right direction. I’m optimistic about a positive outcome,” he said.

